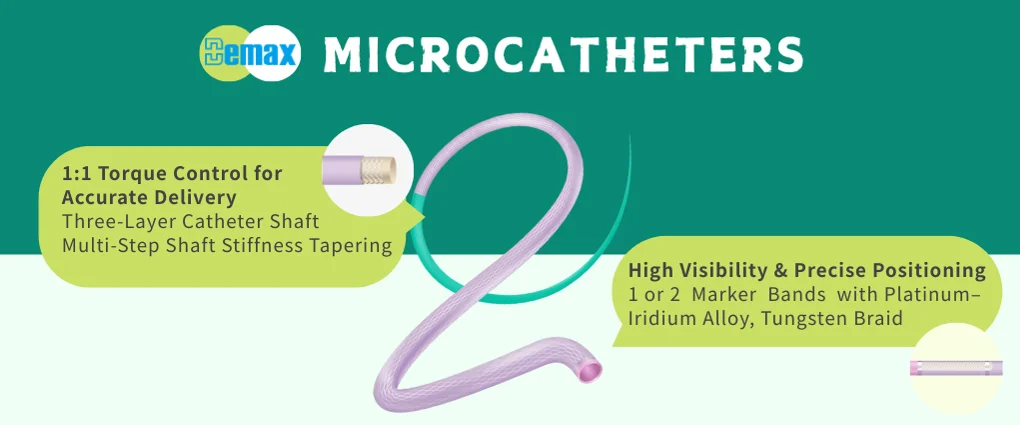
In healthcare manufacturing, the choice of a Medical extrusion tubing supplier shapes device performance, regulatory success, and the real cost of every shipped unit. For Demax, that decision is never a coin toss between “cheap” and “safe.” We build for reliability first, then remove waste everywhere it hides – from resin selection and tooling to documentation and logistics. This article explains how to strike a durable balance between price and assurance, and how Demax‘s ODM approach helps you get there without surprises. Why Cost And Quality Both Matter Cutting visible costs can inflate invisible ones. When tubing wall thickness drifts or durometer varies, downstream yields slide, cycle times stretch, and line operators fight avoidable rework. Adhesive bonds lose strength; kink resistance dips; burst pressure misses the spec. Each deviation triggers extra inspections, line stoppages, and nonconformance reports. The dollars show up later as missed takt, scrapped assemblies, and delayed shipments. Documentation gaps add another layer of risk. Late PPAPs, partial CoCs, or weak change control do not merely frustrate auditors – they slow launches, complicate submissions, and erode customer confidence. Logistics can amplify the pain: long lead times, rigid MOQs, and resin volatility all ripple through planning and inventory. A dependable Medical extrusion tubing supplier prevents those compounding losses. At Demax, we treat quality as the shortest path to lower total cost. We start with the clinical use case and design for manufacturability from day one. Materials are chosen for sterilization method, biocompatibility, and shelf life, not just initial price. Tolerance stacks are modeled for OD/ID, lumen geometry, radiopacity, and surface behavior so the tube you qualify is the tube you keep receiving at scale. The outcome is simple: less scrap, stable takt, and a launch schedule that holds. The Real Price Of “Cheap” Tubing A few cents saved per meter can become dollars lost per device. Subtle issues – gel specks, particulates, inconsistent annealing – can trigger property drift after sterilization or during aging. Demax limits that risk through traceable materials, controlled extrusion windows, and data-backed verification. You should never pay for the same tubing twice. How Demax Delivers Value Without Cutting Corners Demax provides world-class medical catheters, medical guide wires, precision tubing, precision injection molds, intelligent manufacturing lines, and full ODM services. Our approach blends advanced engineering with practical factory discipline so you don’t have to choose between performance and predictability. Design To Verification: Building In Reliability We co-create with your team, translating clinical intent into measurable specifications. Early DFM and design reviews reduce late changes that destroy timelines and budgets. Rapid prototype sample production proves feasibility quickly and clarifies true cost drivers – geometry limits, material interactions, secondary processes – before you lock the design. Under our ISO 13485 quality system, we verify components, assemblies, and finished products for safety and performance, ensuring the documentation package supports submissions and audits. Demax’s ODM model covers the entire journey: concept, design engineering, risk analysis, verification, validation, and transition to manufacturing. The goal is consistent: eliminate unknowns, show the data, and move at a steady, confident pace. Manufacturing Discipline, Data,